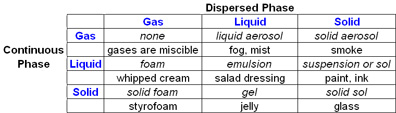
A colloid is typically a two phase system consisting of a continuous phase (the dispersion medium) and dispersed phase (the particles or emulsion droplets). The particle size of the dispersed phase typically ranges from 1 nm to 1000 nm. Examples of colloidal dispersion include solid/liquid (suspensions), liquid/liquid (emulsions), and gas/liquid (foams). A more complete range of colloidal dispersion is shown in the table below.
Particle Interactions
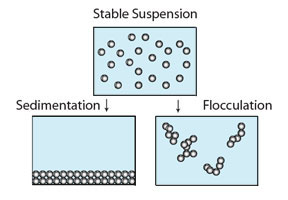
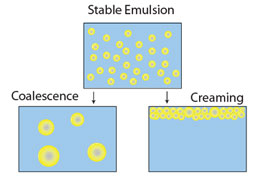
As particle size decreases, surface area increases as a function of total volume. In the colloidal size range there is much interest in particle-particle interactions. Most colloidal commercial products are designed to remain in a stable condition for a defined shelf life. Milk is an example where homogenization is used to reduce droplet size to delay the onset of phase separation (i.e., creaming with the fat rising to the surface). Commercial suspensions may be formulated to keep particles in suspension without sedimenting to the bottom. Examples of phase separation mechanisms are shown below.