Oxidative stress
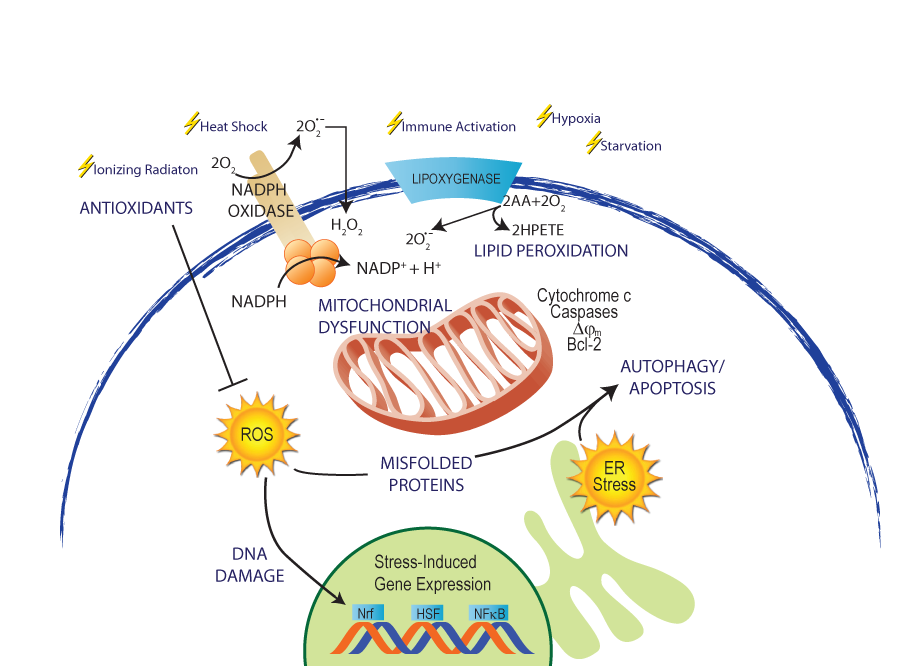
Oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants.
What are free radicals?


A free radicals is an oxygen containing molecule that has one or more unpaired electrons, making it highly reactive with other molecules.
Oxygen by-products are relatively unreactive but some of these can undergo metabolism within the biological system to give rise to these highly reactive oxidants. Not all reactive oxygen species are harmful to the body. Some of them are useful in killing invading pathogens or microbes.
However, free radicals can chemically interact with cell components such as DNA, protein or lipid and steal their electrons in order to become stabilized. This, in turn, destabilizes the cell component molecules which then seek and steal an electron from another molecule, therefore triggering a large chain of free radical reactions.
What are antioxidants?
Every cell that utilizes enzymes and oxygen to perform functions is exposed to oxygen free radical reactions that have the potential to cause serious damage to the cell. Antioxidants are molecules present in cells that prevent these reactions by donating an electron to the free radicals without becoming destabilized themselves. An imbalance between oxidants and antioxidants is the underlying basis of oxidative stress.
Damaged caused by oxidative stress
Oxidative stress leads to many pathophysiological conditions in the body. Some of these include neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, gene mutations and cancers, chronic fatigue syndrome, fragile X syndrome, heart and blood vessel disorders, atherosclerosis, heart failure, heart attack and inflammatory diseases.
| Oxidants | Description |
| •O2-, superoxide anion | One-electron reduction state of O2, formed in many autoxidation reactions and by the electron transport chain. Rather unreactive but can release Fe2+ from iron-sulfur proteins and ferritin. Undergoes dismutation to form H2O2 spontaneously or by enzymatic catalysis and is a precursor for metal-catalyzed •OH formation. |
| H2O2, hydrogen peroxide | Two-electron reduction state, formed by dismutation of •O2- or by direct reduction of O2. Lipid soluble and thus able to diffuse across membranes. |
| •OH, hydroxyl radical | Three-electron reduction state, formed by Fenton reaction and decomposition of peroxynitrite. Extremely reactive, will attack most cellular components |
| ROOH, organic hydroperoxide | Formed by radical reactions with cellular components such as lipids and nucleobases. |
| RO•, alkoxy and ROO•, peroxy radicals | Oxygen centred organic radicals. Lipid forms participate in lipid peroxidation reactions. Produced in the presence of oxygen by radical addition to double bonds or hydrogen abstraction. |
| HOCl, hypochlorous acid | Formed from H2O2 by myeloperoxidase. Lipid soluble and highly reactive. Will readily oxidize protein constituents, including thiol groups, amino groups and methionine. |
| ONOO-, peroxynitrite | Formed in a rapid reaction between •O2- and NO•. Lipid soluble and similar in reactivity to hypochlorous acid. Protonation forms peroxynitrous acid, which can undergo homolytic cleavage to form hydroxyl radical and nitrogen dioxide. |

Leave a Reply
Want to join the discussion?Feel free to contribute!